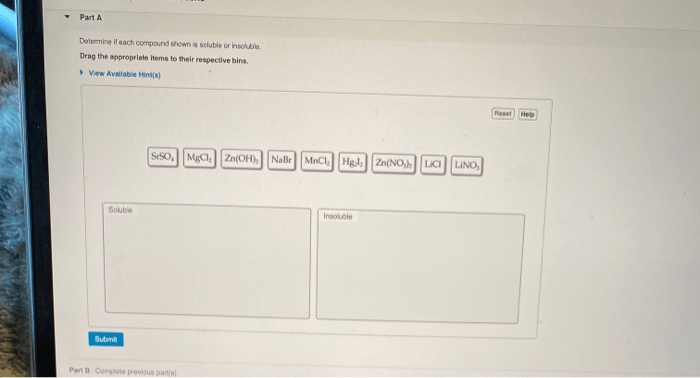
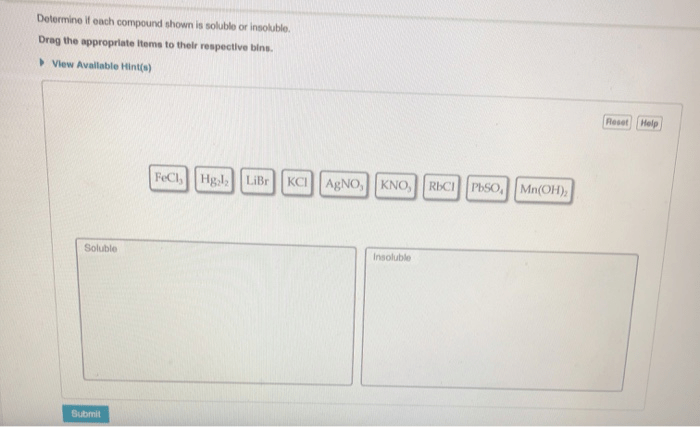
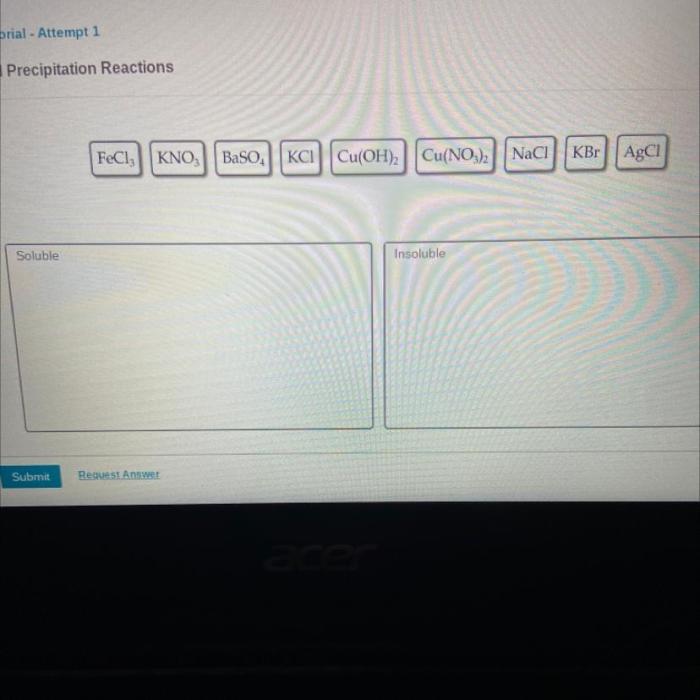
Determine if each compound shown is soluble or insoluble, a fundamental concept in chemistry, unveils the intriguing relationship between molecular structure and its ability to dissolve in various solvents. Understanding solubility is crucial for a myriad of applications, from drug formulations to environmental processes.
Delving into the factors influencing solubility, we explore the impact of temperature, pressure, and pH on the dissolution behavior of compounds. By examining common soluble and insoluble compounds, we uncover the underlying principles governing their solubility characteristics.
Understanding Solubility
Solubility is the ability of a substance to dissolve in a solvent, forming a homogeneous mixture. Key factors influencing solubility include:
- Polarity of the solute and solvent
- Molecular structure of the solute
- Temperature
- Pressure
Polarity refers to the separation of electrical charges within a molecule. Polar molecules have a positive end and a negative end, while nonpolar molecules have a uniform distribution of charge. Like dissolves like, meaning polar solutes dissolve in polar solvents, and nonpolar solutes dissolve in nonpolar solvents.
Evaluating Compound Solubility
The relationship between molecular structure and solubility is complex. Generally, smaller molecules with simpler structures are more soluble than larger, more complex molecules. Ionic compounds tend to be soluble in polar solvents, while covalent compounds tend to be soluble in nonpolar solvents.
Solubility rules can be used to predict the solubility of a compound based on its chemical formula:
- All Group 1 cations (Li+, Na+, K+, Rb+, Cs+) are soluble.
- All Group 2 cations (Ca2+, Sr2+, Ba2+) are soluble, except for BaSO4.
- All ammonium (NH4+) cations are soluble.
- All nitrate (NO3-) anions are soluble.
- All chloride (Cl-) anions are soluble, except for AgCl, PbCl2, and Hg2Cl2.
- All bromide (Br-) anions are soluble, except for AgBr, PbBr2, and Hg2Br2.
- All iodide (I-) anions are soluble, except for AgI, PbI2, and Hg2I2.
- All sulfate (SO42-) anions are soluble, except for BaSO4 and SrSO4.
- All carbonate (CO32-) anions are insoluble, except for Na2CO3, K2CO3, and CaCO3.
- All phosphate (PO43-) anions are insoluble, except for Na3PO4, K3PO4, and NH43PO4.
- All hydroxide (OH-) anions are insoluble, except for NaOH, KOH, Ca(OH)2, Sr(OH)2, and Ba(OH)2.
Common Soluble and Insoluble Compounds, Determine if each compound shown is soluble or insoluble
Soluble Compounds
- NaCl (sodium chloride)
- KBr (potassium bromide)
- NH4Cl (ammonium chloride)
- Na2SO4 (sodium sulfate)
- KNO3 (potassium nitrate)
Insoluble Compounds
- BaSO4 (barium sulfate)
- CaCO3 (calcium carbonate)
- AgCl (silver chloride)
- Fe(OH)3 (iron(III) hydroxide)
- AlPO4 (aluminum phosphate)
Factors Affecting Solubility
Temperature
For most solids, solubility increases with increasing temperature. This is because the increased thermal energy overcomes the intermolecular forces holding the solid together.
Pressure
For gases, solubility increases with increasing pressure. This is because the increased pressure forces more gas molecules into the solvent.
pH
For weak acids and bases, solubility is affected by pH. The solubility of a weak acid increases with increasing pH, while the solubility of a weak base decreases with increasing pH.
Applications of Solubility
Solubility has numerous applications in everyday life and industry:
- Cleaning products: Many cleaning products contain surfactants, which are molecules that increase the solubility of dirt and grime.
- Drug formulations: The solubility of drugs is crucial for their bioavailability and effectiveness.
- Environmental processes: Solubility plays a key role in the transport and fate of pollutants in the environment.
Query Resolution: Determine If Each Compound Shown Is Soluble Or Insoluble
What is the key factor determining compound solubility?
The polarity of the compound and the solvent.
How does temperature affect solubility?
Generally, solubility increases with increasing temperature for solids and decreases for gases.
What is the role of pH in solubility?
pH can affect the solubility of ionic compounds by altering their charge and interactions with the solvent.