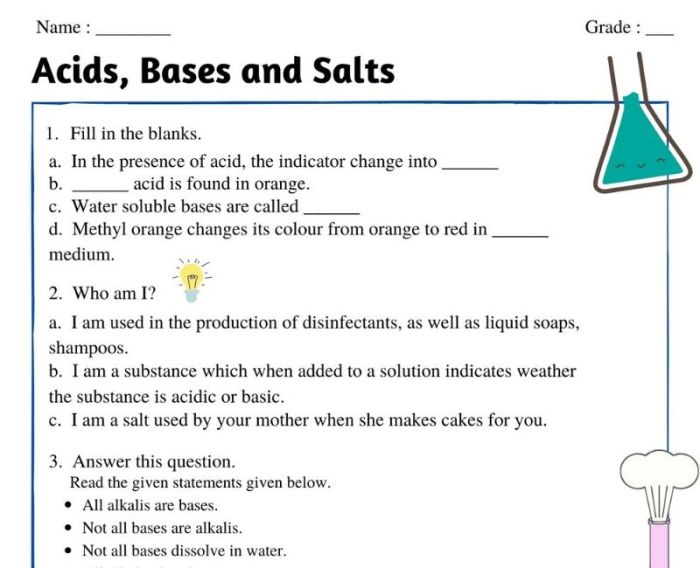
Embark on a captivating journey with our Acid Bases and Salts Worksheet, where we unravel the intriguing world of chemistry. This comprehensive guide provides a thorough exploration of these essential substances, equipping you with a deep understanding of their properties, reactions, and countless applications.
As we delve into the fascinating realm of acids, bases, and salts, you’ll discover their unique characteristics, explore their diverse reactions, and uncover their widespread uses in various industries and everyday life. Prepare to be immersed in a world of chemical reactions and scientific wonders!
Introduction
Acids, bases, and salts are three important types of chemical compounds that play a crucial role in various chemical reactions and processes. Understanding their properties and behavior is essential for comprehending the fundamentals of chemistry.Acids are substances that release hydrogen ions (H+) when dissolved in water.
They typically have a sour taste and can react with metals to produce hydrogen gas. Examples of acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and citric acid (C6H8O7).Bases, on the other hand, are substances that release hydroxide ions (OH-) when dissolved in water.
They have a bitter taste and can feel slippery to the touch. Examples of bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH3).Salts are ionic compounds that are formed when an acid reacts with a base. They are typically neutral in pH and do not undergo ionization in water.
Examples of salts include sodium chloride (NaCl), potassium sulfate (K2SO4), and calcium carbonate (CaCO3).
Properties of Acids, Bases, and Salts: Acid Bases And Salts Worksheet
Acids, bases, and salts exhibit distinct physical and chemical properties that help us differentiate between them. Understanding these properties is crucial for comprehending their behavior in various chemical reactions.
Physical Properties
- Acids:Typically liquids at room temperature, colorless or slightly colored, and have a sour taste.
- Bases:Often solids or liquids, usually white or colorless, and have a bitter taste.
- Salts:Generally solids, can be colorless or colored, and do not have a distinctive taste.
Chemical Properties
- Acids:React with bases to form salts and water, turn blue litmus paper red, and have a pH less than 7.
- Bases:React with acids to form salts and water, turn red litmus paper blue, and have a pH greater than 7.
- Salts:Do not react with acids or bases, do not change the color of litmus paper, and have a pH of 7.
Table of Properties
| Property | Acids | Bases | Salts |
|---|---|---|---|
| Physical State | Liquid (mostly) | Solid/Liquid | Solid (mostly) |
| Taste | Sour | Bitter | No distinct taste |
| Litmus Paper | Turns red | Turns blue | No change |
| pH | < 7 | > 7 | = 7 |
| Reactivity | React with bases | React with acids | No reactivity |
Reactions of Acids, Bases, and Salts
Acids, bases, and salts undergo various types of reactions, each with its own characteristics and applications.
Neutralization Reactions
Neutralization reactions occur when an acid and a base react to form a salt and water. These reactions are typically exothermic, releasing heat.
After finishing the acid bases and salts worksheet, you might want to try something different. I highly recommend checking out the tragedy of the commons lab for an engaging and interactive experience. When you’re done with that, you can always come back to the acid bases and salts worksheet for more practice.
- Example: Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water (H 2O):
HCl + NaOH → NaCl + H2O
Precipitation Reactions, Acid bases and salts worksheet
Precipitation reactions occur when two solutions containing ions react to form an insoluble solid precipitate. These reactions are often used to separate and purify substances.
- Example: Silver nitrate (AgNO 3) reacts with sodium chloride (NaCl) to form silver chloride (AgCl) precipitate and sodium nitrate (NaNO 3):
AgNO3+ NaCl → AgCl (precipitate) + NaNO 3
Acid-Base Reactions
Acid-base reactions involve the transfer of protons (H +ions) between an acid and a base. These reactions can be used to determine the strength of acids and bases.
- Example: Acetic acid (CH 3COOH) reacts with sodium hydroxide (NaOH) to form sodium acetate (CH 3COONa) and water (H 2O):
CH3COOH + NaOH → CH 3COONa + H 2O
Applications of Acids, Bases, and Salts
Acids, bases, and salts play crucial roles in various aspects of our daily lives and industrial processes. Their unique chemical properties make them indispensable in a wide range of applications, from food preservation to medical treatments and manufacturing.
Household Applications
- Vinegar (acetic acid): A common household cleaner and disinfectant due to its acidic nature, killing bacteria and removing stains.
- Baking soda (sodium bicarbonate): A base used as a leavening agent in baking, creating carbon dioxide gas that causes baked goods to rise.
- Table salt (sodium chloride): A salt essential for seasoning food and preserving it by inhibiting bacterial growth.
Industrial Applications
- Sulfuric acid: A strong acid used in the production of fertilizers, dyes, and batteries.
- Sodium hydroxide: A strong base used in soap and paper manufacturing, as well as in drain cleaners.
- Calcium carbonate: A salt used as a filler in paper, paint, and plastics, and as an antacid to neutralize stomach acid.
Medical Applications
- Citric acid: An acid found in citrus fruits, used as a preservative and flavoring agent in food and beverages, and as a component in effervescent tablets.
- Aspirin (acetylsalicylic acid): An acidic drug used as a pain reliever and anti-inflammatory agent.
- Antacids: Bases used to neutralize stomach acid, relieving heartburn and indigestion.
Safety Considerations
When working with acids, bases, and salts, it is crucial to adhere to safety precautions to prevent accidents and protect your health. These substances possess inherent hazards that require careful handling and appropriate protective measures.
Acids
- Acids are corrosive and can cause severe burns to the skin and eyes. They can also release toxic fumes that are harmful to inhale.
- Wear protective clothing, including gloves, eye protection, and a lab coat, when handling acids.
- Never mix concentrated acids with water directly, as this can cause a violent reaction and splatter.
- Handle acids in a well-ventilated area to avoid exposure to harmful fumes.
Bases
- Bases can also cause skin and eye irritation, and some are corrosive. They can be harmful if ingested.
- Wear protective gear similar to that used for acids, including gloves, eye protection, and a lab coat.
- Avoid contact with strong bases, as they can cause severe burns.
- Neutralize bases with an acid before disposing of them to prevent environmental damage.
Salts
- Salts are generally less hazardous than acids and bases, but some can be toxic or corrosive.
- Read the Material Safety Data Sheet (MSDS) for specific salts to determine their potential hazards.
- Wear gloves and eye protection when handling salts, especially if they are in powder form.
- Avoid inhaling salt dust, as it can irritate the respiratory tract.
Expert Answers
What is the difference between an acid and a base?
Acids are substances that release hydrogen ions (H+) when dissolved in water, while bases release hydroxide ions (OH-) when dissolved in water.
What is the pH scale?
The pH scale measures the acidity or alkalinity of a solution. It ranges from 0 to 14, with 0 being the most acidic and 14 being the most alkaline (basic).
What is a neutralization reaction?
A neutralization reaction is a chemical reaction between an acid and a base that produces a salt and water.